Vascular Metabolism
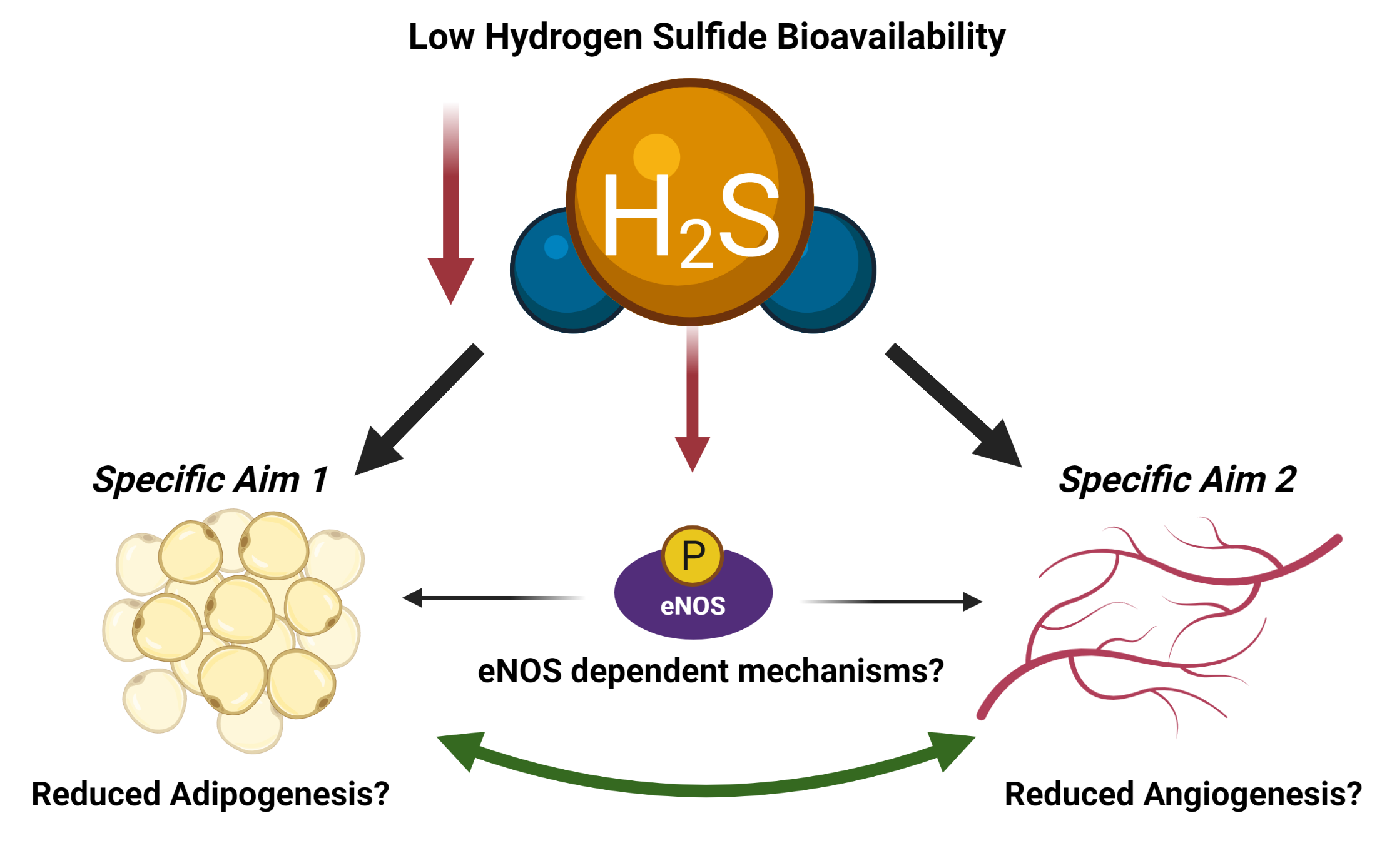
Insulin resistance is a hallmark of the pathogenesis from obesity to type 2 diabetes and is intimately involved in vascular dysfunction. Adipose tissue is a highly vascularized organ with each adipocyte in contact with a blood vessel. The function of the adipose tissue endothelium is critical for the function of the adipocyte and vice versa. In genetic and diet-induced models of obesity, reduced angiogenesis is mirrored by a reduction in adipogenesis, the formation of new adipocytes. In conditions of obesity and/or insulin resistance, adipocytes become less vascularized which diminishes their capacity to regulate lipid storage and release. Increased circulating free fatty acids results in ectopic storage lipid deposition and vascular inflammation. The vascular endothelial cells and adipocytes share the burden of obesity; therefore, it is critical to understand how these cells communicate their respective metabolic/redox states to maintain healthy adipose tissue function.
A unifying phenomenon linking the pathology of obesity-associated metabolic-vascular disease is the reduced bioavailability of gaseous signaling molecules (gasotransmitters) such as nitric oxide (NO) and hydrogen sulfide (H2S). In this COBRE project, we will determine the role of hydrogen sulfide in endothelial-to-adipocyte communication. This project will involve innovative methods and approaches to study animal models of deficient hydrogen sulfide bioavailability.